NHS North West Genomics
0.2.1 - ci-build
NHS North West Genomics
0.2.1 - ci-build
NHS North West Genomics - Local Development build (v0.2.1) built by the FHIR (HL7® FHIR® Standard) Build Tools. See the Directory of published versions
| Official URL: https://fhir.nwgenomics.nhs.uk/ImplementationGuide/fhir.nwgenomics.nhs.uk | Version: 0.2.1 | |||
| Draft as of 2026-02-15 | Computable Name: NWGMSA | |||
Diagnostic testing is essential to modern clinical care, offering objective information that supports decision-making at every stage of a patient’s journey—from initial evaluation to long-term monitoring and assessment of outcomes.
Genomic diagnostic testing contributes to this process by examining a patient’s DNA or RNA to detect genetic variations that influence disease susceptibility, diagnosis, treatment choices, and prognosis. By delivering highly specific and personalised insights, genomic testing improves the accuracy and effectiveness of clinical management.
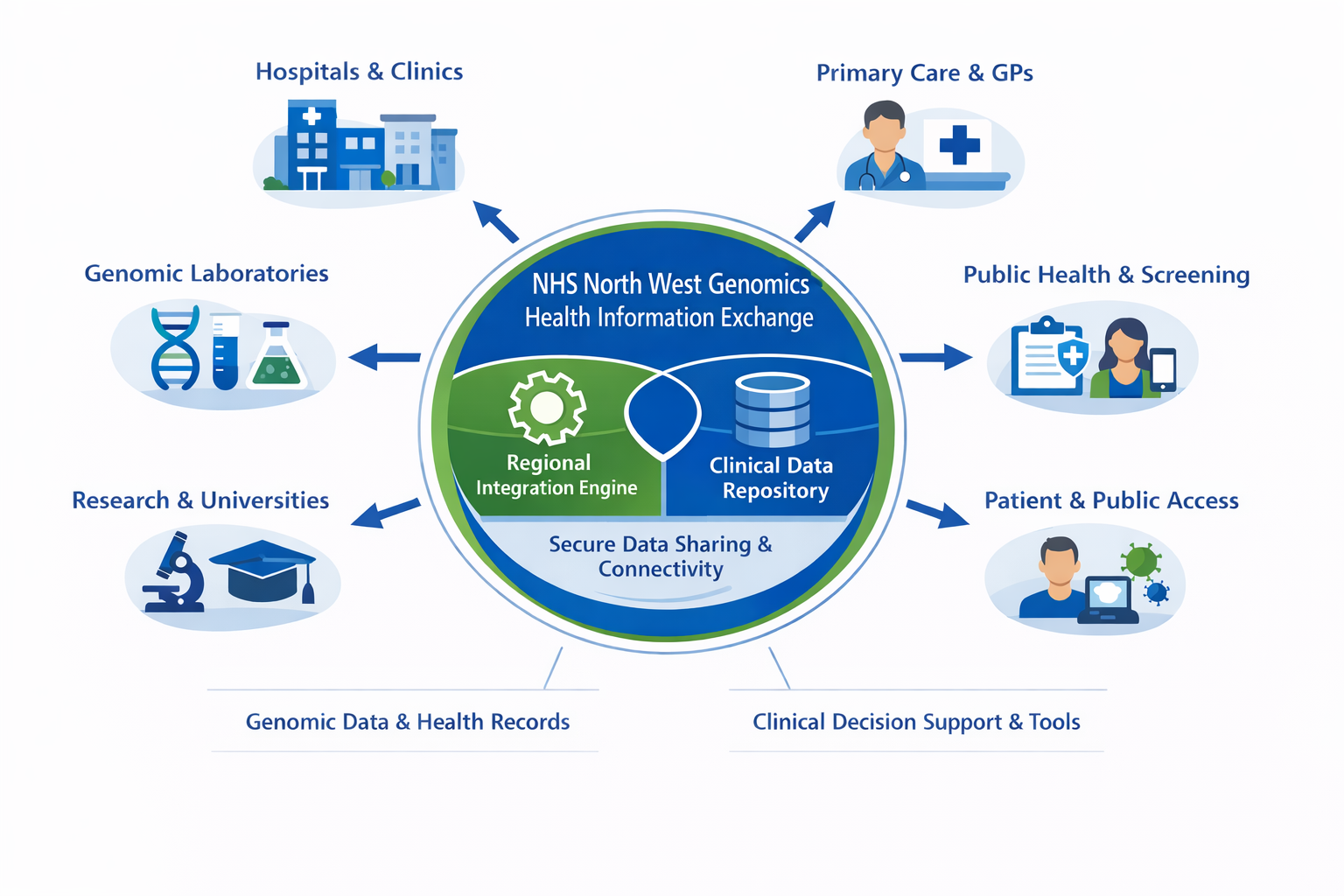
NHS North West Genomics is a new regional NHS service that consolidates clinical genomic testing across the North West of England. Although the service is delivered regionally, it also processes genomic test requests from across the UK. The service is hosted by Manchester University NHS Foundation Trust.
As part of the service transition, existing systems for electronic test ordering and reporting will be enhanced through the introduction of a Regional Integration Engine (RIE) and a Genomic Clinical Data Repository. These components enable seamless data exchange between local clinical systems and regional genomic laboratory services.
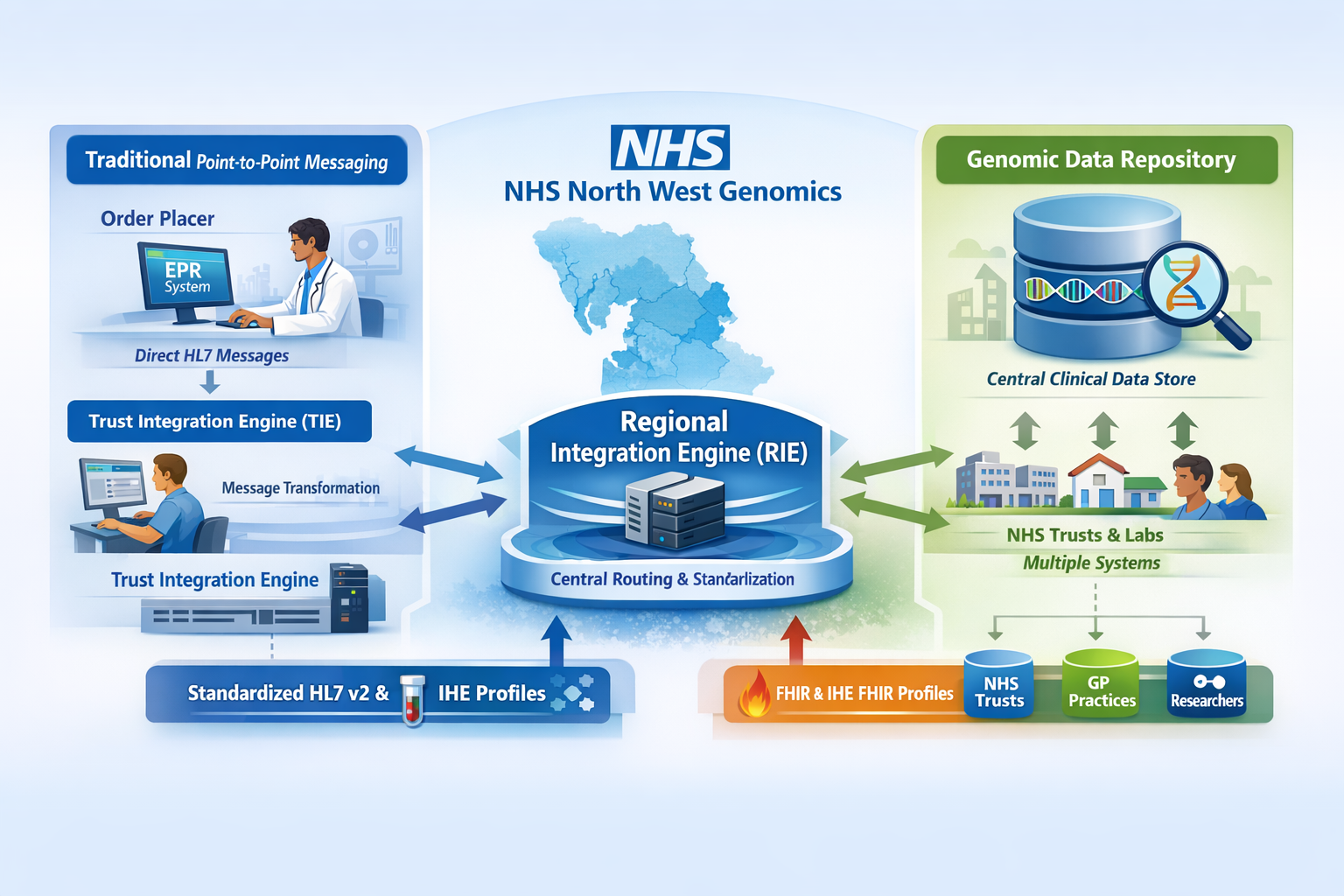
The diagram below illustrates point to point messaging between an Order Placer and an Order Filler. The Order Filler is typically a Laboratory Information Management System (LIMS), while the Order Placer is usually a clinical system such as an Electronic Patient Record (EPR).
Not all interactions will necessarily be electronic. For example, reports may be sent by email, and orders may be submitted via email or physically accompany the specimen.
graph LR
OrderPlacer[<b>Order Placer</b><br/>EPR] --> |1. General Order<br/>HL7 v2 ORM_O01| OrderFiller
OrderFiller[<b>Order Filler</b><br/>LIMS] --> |2. Laboratory Report<br/>HL7 v2 ORU_R01| OrderPlacer
classDef purple fill:#E1D5E7;
class OrderPlacer,OrderFiller purple
In many NHS Trusts, a Trust Integration Engine (TIE) is used to facilitate this point-to-point messaging.
graph LR
OrderPlacer[<b>Order Placer</b><br/>EPR] --> |1. General Order<br/>HL7 v2 ORM_O01| TIE["Trust Integration Engine (TIE)"]
TIE --> |2. General Order<br/>HL7 v2 ORM_O01| OrderFiller
OrderFiller[<b>Order Filler</b><br/>LIMS] --> |3. Laboratory Report<br/>HL7 v2 ORU_R01| TIE
TIE --> |4. Laboratory Report<br/>HL7 v2 ORU_R01| OrderPlacer
classDef purple fill:#E1D5E7;
class OrderPlacer,OrderFiller purple
TIEs typically handle transformations between the different HL7 v2 variants used by Order Placers (e.g. EPRs) and Order Fillers (e.g. LIMS).
Existing interfaces to NW Genomics LIMS will be migrated to use the Regional Integration Engine (RIE). The RIE performs similar functions to NHS Trust TIEs, and in the interim phase will perform pass through routing of messages only.
graph LR
OrderPlacer[<b>Order Placer</b><br/>EPR] --> |1. General Order<br/>HL7 v2 ORM_O01| TIE["Trust Integration Engine (TIE)"]
TIE --> |2. General Order<br/>HL7 v2 ORM_O01| RIE
RIE["Regional Integration Engine (RIE)"] --> |3. General Order<br/>HL7 v2 ORM_O01| OrderFiller
OrderFiller[<b>Order Filler</b><br/>LIMS] --> |4. Laboratory Report<br/>HL7 v2 ORU_R01| RIE
RIE --> |5. Laboratory Report<br/>HL7 v2 ORU_R01| TIE
TIE --> |6. Laboratory Report<br/>HL7 v2 ORU_R01| OrderPlacer
classDef purple fill:#E1D5E7;
class OrderPlacer,OrderFiller purple
The regional genomic service supports more than 20 NHS Trusts, each potentially operating different clinical systems. Within NHS North West Genomics itself, multiple LIMS and supporting clinical systems are in use. Under a traditional point-to-point integration model, this rapidly leads to a highly complex and fragile integration landscape.
graph LR
OrderPlacerA[<b>Order Placer</b><br/>NHS Trust A]
OrderPlacerB[<b>Order Placer</b><br/>NHS Trust B]
OrderPlacerC[<b>Order Placer</b><br/>NHS Trust C]
LIMSA[<b>Order Filler</b><br>LIMS iGene]
LIMSB[<b>Order Filler</b><br>LIMS B]
LIMSC[<b>Order Filler</b><br>LIMS C]
LIMSD[<b>Order Filler</b><br>LIMS D]
OrderPlacerA <--> LIMSA
OrderPlacerA <--> LIMSB
OrderPlacerA <--> LIMSC
OrderPlacerA <--> LIMSD
OrderPlacerB <--> LIMSA
OrderPlacerB <--> LIMSB
OrderPlacerB <--> LIMSC
OrderPlacerB <--> LIMSD
OrderPlacerC <--> LIMSA
OrderPlacerC <--> LIMSB
OrderPlacerC <--> LIMSC
OrderPlacerC <--> LIMSD
To address this challenge, the RIE acts as a central routing and interoperability hub. All orders and reports are exchanged between NHS Trust ordering systems and North West Genomics LIMS platforms via the RIE.
Rather than maintaining multiple bespoke integrations, each participant integrates once with the RIE. Trust Integration Engines remain responsible for transforming messages between local EPR systems and the regional standard used by the RIE.
A key element of this approach is the introduction of standardised message formats and interactions between Trust TIEs and the RIE. These standards are based on a single, shared data model known as the Data Contract.
graph LR
subgraph NHSTrustA[NHS Trust A]
NHSA[<b>Order Placer</b><br/>EPR]
end
subgraph NHSTrustB[NHS Trust B]
NHSB[<b>Order Placer</b><br/>EPR]
end
subgraph DataContracts[Data Contract]
TIEA["NHS Trust A Integration Engine (TIE)"]
TIEB["NHS Trust B Integration Engine (TIE)"]
RIE["Regional Integration Engine (RIE)"]
end
NHSA --> |Laboratory Order<br/>HL7 ORM_O01| TIEA
NHSB --> |Laboratory Order<br/>HL7 OML_O21| TIEB
TIEA --> |Laboratory Order<br/>HL7 OML_O21<br/>HE LTW LAB-1| RIE
TIEB --> |Laboratory Order<br/>HL7 OML_O21<br/>HE LTW LAB-1| RIE
NHSB --> RIE
RIE --> |Laboratory Order<br/>HL7 v2 OML_O21| LIMSA
RIE --> LIMSB
RIE --> LIMSC
RIE --> LIMSD
LIMSA[<b>Order Filler</b><br>LIMS iGene]
LIMSB[<b>Order Filler</b><br>LIMS Shire]
LIMSC[<b>Order Filler</b><br>LIMS C]
LIMSD[<b>Order Filler</b><br>LIMS D]
Equivalent patterns apply to laboratory reports.
graph LR
subgraph NHSTrustA[NHS Trust A]
NHSA[<b>Order Placer</b><br/>EPR]
end
subgraph NHSTrustB[NHS Trust B]
NHSB[<b>Order Placer</b><br/>EPR]
end
subgraph DataContracts[Data Contract]
RIE["Regional Integration Engine (RIE)"]
TIEA["NHS Trust A Integration Engine (TIE)"]
TIEB["NHS Trust B Integration Engine (TIE)"]
end
ICSA[NHS ICS A]
ICSB[NHS ICS B]
APPA[NW Genomics Application 1]
APPB[NW Genomics Application 2]
LIMSA[<b>Order Filler</b><br>LIMS iGene]
LIMSB[<b>Order Filler</b><br>LIMS Shire]
LIMSC[<b>Order Filler</b><br>LIMS C]
LIMSD[<b>Order Filler</b><br>LIMS D]
LIMSA --> |Laboratory Report<br/>HL7 v2 ORU_R01| RIE
LIMSB --> RIE
LIMSC --> RIE
LIMSD --> RIE
RIE["Regional Integration Engine (RIE)"] --> |Laboratory Report<br/>HL7 v2 ORU_R01<br/>IHE LTW LAB-3| TIEA
RIE --> |Laboratory Report<br/>HL7 v2 ORU_R01<br/>IHE LTW LAB-3| TIEB
TIEA --> |Laboratory Report<br/>HL7 v2 ORU_R01| NHSA
TIEB --> |Laboratory Report<br/>HL7 v2 ORU_R01| NHSB
RIE --> |Document Notification<br/>HL7 MDM_T02<br/>IHE XDS/MHD| ICSA
RIE --> ICSB
RIE --> |Laboratory Report<br/>HL7 v2 ORU_R01<br/>IHE LTW LAB-3| APPB
RIE --> APPA
The primary distinction between a Regional Integration Engine and a Trust Integration Engine is scope:
This hub-and-spoke model significantly reduces integration complexity, improves maintainability, and supports consistent data quality across the region. The main distinction between a Regional Integration Engine (RIE) and a Trust Integration Engine is that the RIE functions as a central routing hub. Each participant connects only to the RIE rather than individually integrating with multiple other systems. This significantly reduces integration complexity. Trust TIEs will still be responsible for transforming messages between their internal EPR systems and the RIE.
Because the RIE operates at a regional level, certain HL7 v2 message components must be standardised or updated. These changes ensure global uniqueness for identifiers such as:
Standard clinical terminologies are used to ensure semantic interoperability:
These requirements are outlined in the Data Contract. HL7 v2 message exchanges are aligned with HL7 v2.5.1 and the following IHE profiles (API Contracts):
From a practical perspective, the RIE is introduced into the existing point-to-point messaging flow. It is at this boundary—between the TIE and the RIE—that the use of the Data Contracts , including HL7 v2.5.1 and adoption of IHE LTW, is mandated.
Data Contracts are not mandated between the RIE and LIMS, nor between the TIE and EPR. For practical reasons, these systems may require changes in the future to align with the central Data Contracts.
NHS Trust TIEs do not interface directly with the LIMS within NHS North West Genomics and, going forward, will not interface directly with LIMS from other regional genomic systems and NHS England Genomic Order Management Service. All such interactions will be managed by the RIE.
graph LR
OrderPlacer[<b>Order Placer</b><br/>EPR] --> |1. General Order<br/>HL7 v2 ORM_O01/OML_O21| TIE
subgraph DataContract[Data Contract]
TIE["Trust Integration Engine (TIE)"]
RIE["Regional Integration Engine (RIE)"]
end
TIE --> |2. General Order<br/>HL7 v2.5.1 OML_O21| RIE
RIE --> |3. General Order<br/>HL7 v2 ORM_O01/OML_O21| OrderFiller
OrderFiller[<b>Order Filler</b><br/>LIMS] --> |4. Laboratory Report<br/>HL7 v2 ORU_R01| RIE
RIE --> |5. Laboratory Report<br/>HL7 v2.5.1 ORU_R01| TIE
TIE --> |6. Laboratory Report<br/>HL7 v2 ORU_R01| OrderPlacer
Traditional messaging focuses solely on communication between two systems—the order placer and the order filler—and does not support wider sharing of genomic data across multiple organisations such as NHS Trusts, GP practices, or other clinical teams.
To address this, a central Genomic Data Repository (GDR) will be established. This repository will provide a read-only FHIR RESTful (read only API) and will be populated via data flows through the RIE (See Health Information Exhange (HIE)) and will focus primarily on sharing data produced by NHS North West Genomics.
graph TD
subgraph DataContracts[Data Contract]
CDR["<b>Data Source</b>Genomic Data Repository (GDR)"]
NHSA[<b>Data Consumer</b><br/>NHS GP/Trust/Board/ICS A]
NHSB[<b>Document Consumer</b><br/>NHS GP/Trust/Board/ICS B]
APPA[<b>Data Consumer</b><br/>Application 1]
APPB[<b>Document Consumer</b><br/>Application 2]
end
NHSE[<b>Document Consumer</b><br/>NHS England<br/>Summary Care Record<br/>National Record Locator]
CDR --> |HL7 FHIR RESTful<br/>IHE QEDm| NHSA
CDR --> |HL7 FHIR RESTful<br/>IHE MHD| NHSB
CDR --> |HL7 FHIR RESTful| NHSE
CDR --> |HL7 FHIR RESTful<br/>IHE QEDm|APPA
CDR --> |HL7 FHIR RESTful<br/>IHE MHD| APPB
classDef purple fill:#E1D5E7;
class CDR,NHSA,NHSB,APPA,APPB,NHSE purple
The Data Contract and data structures used in the FHIR interfaces follow the same conventions as those used in the HL7 v2 message exchanges.
The CDR is expected to adopt emerging IHE Europe standards for clinical data and document sharing. These currently include (API Contract):
The introduction of data and document sharing using HL7 FHIR RESTful APIs enables a transition from a traditional message-based workflow to an event-based workflow (see FHIR Workflow).
graph LR
subgraph OrderPlacerM[Order Placer]
OrderPlacer[<b>Order Placer</b><br/>EPR]
DataO["<b>Data Source and Consumer</b>"]
end
subgraph OrderFillerM[Order Filler]
OrderFiller[<b>Order Filler</b><br/>LIMS]
DataF["<b>Data Source and Consumer</b>"]
end
OrderPlacer--> |Event Notification - FHIR Task| OrderFiller
OrderFiller --> |Event Notification - FHIR Task| OrderPlacer
DataO --> |HL7 FHIR RESTful<r/>IHE QEDm/MHD/PDQm| DataF
DataF --> |HL7 FHIR RESTful<r/>IHE QEDm/MHD/PDQm| DataO
classDef purple fill:#E1D5E7;
class OrderPlacer,OrderFiller,DataO,DataF purple
In this model, orders and reports are no longer exchanged directly between the Order Placer and the Order Filler. Instead, both systems communicate through FHIR Tasks, using event notifications to coordinate activities and state changes.
This represents a shift from a message-oriented workflow (see EIP Messaging Patterns) to a conversation-based workflow (see EIP Conversation Patterns). Rather than relying on single, transactional messages, the workflow is managed as an ongoing conversation between participants.
Although this approach involves multiple exchanges between the Order Placer and the Order Filler, it more accurately reflects real-world clinical workflows, where work progresses through a series of coordinated steps, acknowledgements, and state transitions rather than a single request–response interaction.
It is anticipated that event notifications will be implemented using FHIR Subscriptions, which support a publish–subscribe (pub/sub) pattern, or alternatively through the NHS England Multicast Notification Service API.
sequenceDiagram
participant OrderPlacer As Order Placer
participant DataO as Data Source <br/> Order Placer
participant OrderFiller As Order Filler
participant DataF as Data Source <br/> Order Filler
OrderPlacer ->> DataO: Create Order
OrderPlacer ->> OrderFiller: DiagnosticRequest - Event Notification (FHIR Task (requested))
OrderFiller ->> DataO: Retrieve Laboratory Order (FHIR RESTful API Query)
alt is accepted
OrderFiller ->> OrderPlacer: Task Diagnostic Request - Event Notification (FHIR Task (accepted))
Note over OrderFiller: Starts Testing
OrderFiller ->> OrderPlacer: Task Diagnostic Request - Event Notification (FHIR Task (in-progress))
Note over OrderFiller: Interpretation of results and write Report
OrderFiller ->> DataF: Creates Laboratory Order
OrderFiller ->> OrderPlacer: Task DiagnosticRequest - Event Notification (FHIR Task (completed))
OrderPlacer ->> DataF : Retrieve Laboratory Report (FHIR RESTful API Query)
else is rejected
OrderFiller ->> OrderPlacer: Task Diagnostic Request - Event Notification (FHIR Task (rejected))
end
This diagram illustrates an event-based, conversation-driven laboratory ordering workflow using HL7 FHIR Tasks.
The Order Placer creates a diagnostic order and notifies the Order Filler via a FHIR Task. The Order Filler retrieves the order using FHIR RESTful queries, accepts or rejects the request, and communicates status updates (accepted, in-progress, completed, or rejected) back to the Order Placer through task-based event notifications. Laboratory testing, result interpretation, and report creation occur asynchronously, with reports retrieved by the Order Placer via FHIR RESTful APIs upon task completion.
A conversational (event-based) workflow, also referred to as a conversation-based workflow, represents a modern approach to clinical messaging. This paradigm assumes that both the Order Placer and the Order Filler can share data using HL7 FHIR RESTful APIs.
In practice, this capability may not always be available. For example, Laboratory Information Management Systems (LIMS) within NHS North West Genomics may not support FHIR RESTful APIs. In such cases, the Genomic Data Repository (GDR) is used to share genomic laboratory reports and other genomic data. Similarly, if Electronic Patient Record (EPR) systems do not support FHIR RESTful APIs, the GDR is used to facilitate the sharing of laboratory orders.
Together, the Regional Integration Engine (RIE) and the Genomic Data Repository (GDR) collectively constitute the Health Information Exchange (HIE).
Health Information Exchange (HIE)
| Menu Item | Description | Audience | |
|---|---|---|---|
| Analysis and Design (Volume 1) | Description of the processes and corresponding technical frameworks | General | |
| Interfaces (Volume 2) | Description of the processes and corresponding technical frameworks (HL7 v2 and FHIR Interactions) | Detailed Technical (Integration Developer) | |
| Data Models (Volume 3) | NHS North West Forms, Templates, Reports, and Compositions | Data Modeling (Detailed Technical) | |
| Artefacts (Volume 4) | NHS North West Common Data Models | Detailed Technical | |
| Development | Testing, Suppport and Architecture | Detailed Technical (Developer) |
UK edition of SNOMED (83821000000107)
Package hl7.fhir.uv.extensions.r4#5.2.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Mon, Feb 10, 2025 21:45+1100+11:00) |
Package hl7.fhir.uv.ipa#1.1.0 This IG describes how an application acting on behalf of a patient can access information about the patient from an clinical records system using a FHIR based API. The clinical records system may be supporting a clinical care provider (e.g. a hospital, or a general practitioner), or a health data exchange, including a national health record system. (built Wed, Mar 19, 2025 14:34+0000+00:00) |
Package hl7.fhir.uv.xver-r5.r4#0.0.1-snapshot-2 The cross-version extensions available in FHIR R4 from FHIR R5 (built Sat, Sep 13, 2025 16:55-0400-04:00) |
Package hl7.fhir.eu.extensions.r4#1.2.0 This guide lists the extensions specified for the European REALM. (built Fri, Dec 19, 2025 11:52+0100+01:00) |
Package ihe.pharm.mpd.r4#1.0.0-comment-2 ImplementationGuide for IHE Pharmacy Medication Prescription and Dispense (MPD) profile (built Tue, May 27, 2025 16:32+0200+02:00) |
Package hl7.fhir.eu.base#2.0.0-ballot This guide collects base and core profiles to be used in the European context. It also includes common artifacts, such as the profiles describing the European Health Insurance Card. (built Fri, Dec 19, 2025 18:28+0100+01:00) |
Package fhir.r4.ukcore.stu3.currentbuild#0.0.19-pre-release UK Core FHIR profiles and Assets |
Package hl7.fhir.uv.extensions.r4#5.1.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Sat, Apr 27, 2024 18:39+1000+10:00) |
Package hl7.fhir.uv.genomics-reporting#3.0.0 Guidelines for reporting of clinical genomics results using HL7 FHIR. (built Thu, Dec 12, 2024 20:34+0000+00:00) |
Package hl7.fhir.uv.ips#1.1.0 International Patient Summary (IPS) FHIR Implementation Guide (built Tue, Nov 22, 2022 03:24+0000+00:00) |
Package hl7.fhir.eu.laboratory#0.1.1 This guide describes how the Laboratory Report can be represented in the European REALM. (built Tue, Mar 25, 2025 12:00+0100+01:00) |
Package hl7.fhir.uv.sdc#3.0.0 The SDC specification provides an infrastructure to standardize the capture and expanded use of patient-level data collected within an EHR. |
Package hl7.fhir.uv.tools.r4#0.9.0 This IG defines the extensions that the tools use internally. Some of these extensions are content that are being evaluated for elevation into the main spec, and others are tooling concerns (built Tue, Dec 16, 2025 23:18+1100+11:00) |
| Role(s) | Contributor(s) |
|---|---|
| North West Genomic Medicine Service Alliance | |
| Alder Hey Children's Hospital Trust | |
| Manchester University NHS Foundation Trust | |
| Liverpool Womens NHS Foundation Trust | |
| The Christie NHS Foundation Trust | |
| NHS England | |
| Staff Engineer | Kevin Mayfield, Aire Logic & Mayfield IS |