NHS North West Genomics
0.1.0 - ci-build
NHS North West Genomics
0.1.0 - ci-build
NHS North West Genomics - Local Development build (v0.1.0) built by the FHIR (HL7® FHIR® Standard) Build Tools. See the Directory of published versions
This guide is to support Genomic Testing Workflow at a regional level and is designed to be compatible with:
The general workflow is based on IHE LTW profiles and HL7 v2 OML and ORU.
Genomic Testing Workflow is part of Diagnostic Testing, which is also part of the general clinical process.
graph TD;
A[Assessment]-->|Creates Observations| B;
A--> |Needs Diagnostic Testing and Completes| T;
B[Diagnosis]-->|Creates Condition| C;
T[<b>Order Placer</b><br/>Genomics Test Order]--> |"Sends Laboratory Order - LAB-1<br/>FHIR Message O21"| AN;
T --> |Asks for| S
S[Specimen Collection] --> |Sends Specimen| AN;
AN["<b>Order Filler</b><br/>Diagnostic Testing"] --> |"Requests further tests <br/>(reflex order)"| T;
AN --> |"Creates Laboratory Report - LAB-3<br/>HL7 v2 ORU_R01"| B;
AN --> |Sends Laboratory Report| A;
C[Plan]-->|Creates Goals and Tasks| D;
D[Implement/Interventions]-->|Actions Tasks| E;
E[Evaluate]--> |Reviews Care| A;
click T Questionnaire-GenomicTestOrder.html
click AN Questionnaire-GenomicTestReport.html
click S ExampleScenario-BiopsyProcedure.html
classDef purple fill:#E1D5E7;
classDef yellow fill:#FFF2CC;
classDef pink fill:#F8CECC
classDef green fill:#D5E8D4;
classDef blue fill:#DAE8FC;
classDef orange fill:#FFE6CC;
class A pink
class B yellow
class C green
class D blue
class E orange
class O,S,T,AN purple
Genomic diagnostic testing follows the same standardized process defined by the IHE Laboratory Testing Workflow used in traditional laboratory testing. This workflow has been enhanced to support the sharing of laboratory reports (documents) through Integrated Care Systems (ICS). In addition, a new mechanism for sharing laboratory reports has been introduced to establish a regional genomic data repository.
graph TD;
Practitioner[fas:fa-user-md Practitioner] --> |1. Selects Order Form| FormManager
FormManager --> OrderEntry
Practitioner --> |3. Completes| OrderEntry[Order Form]
EPR[fas:fa-database Electronic Patient Record] --> |2. Pre Populates with existing data| OrderEntry
OrderEntry --> |4. Submits Order| EPR
EPR --> |5. Sends Laboratory Order| DiagnosticTesting[fas:fa-stethoscope Diagnostic Testing]
For more details see:
graph TD;
DiagnosticTesting[fas:fa-stethoscope Diagnostic Testing] --> |Creates| DiagnosticReport[Diagnostic Report]
DiagnosticReport --> |Reads Variant and DiagnosticImplication| Consultant[fas:fa-user-md Consultant]
DiagnosticReport --> |Reads Variant and DiagnosticImplication| GP[fas:fa-user-md GP]
EPR --> |GC1. Reads Variant and DiagnosticImplication| GeneticCounseling[fas:fa-user-md Genetic Counseling]
DiagnosticReport --> |Reads Genomic Study and Variant| Oncologist[fas:fa-user-md Oncologist]
DiagnosticReport --> |Is Persisted| GenomicCDR[fas:fa-database Genomic Clinical Data Repository]
DiagnosticReport --> |"Reads DiagnosticImplication (Condition) and Variant (Gene)"| Patient[fas:fa-user Patient]
Consultant --> |"Records Condition (from Diagnostic Implication)"| EPR[fas:fa-database Electronic Patient Record]
GP--> |"Records Condition (Genomic Disorder) <br/>or Observation (Genomic Disorder Carrier) <br/>based on Diagnostic Implication"| GPEPR[fas:fa-database GP Electronic Patient Record]
GeneticCounseling --> |GC2. Obtains Family Structure and History| Patient
GeneticCounseling --> |GC3. Records FamilyMemberHistory| EPR
Consultant --> |Asks for Genetic Counseling on Genetic Condition?| GeneticCounseling
A detailed example of this process can be found in the Example Scenario - Clinical and Genomic Workflow.
For more details see:
For illustration purposes only, see Inter Laboratory Workflow
For illustration purposes only, see Specimen Event Tracking
The data model used in this guide is a combination of data and workflow requirements from a variety of other guides.
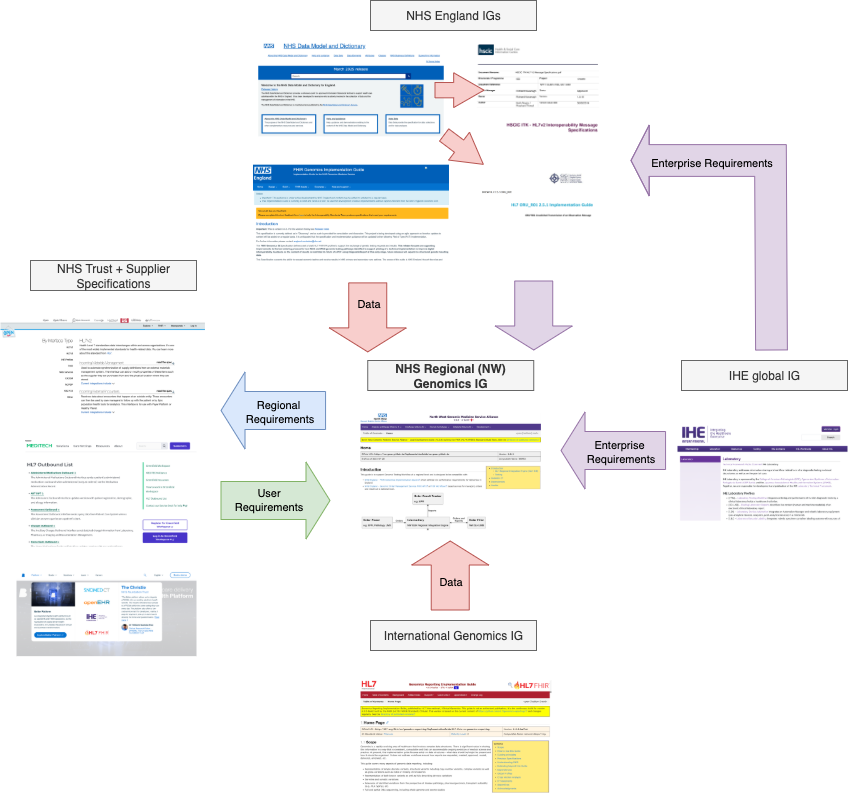
North West GMSA IG
This implementation guide will also enable use of FHIR Testing tools such as Command Line FHIR Validation and Online FHIR Validation